Learn More
Genscript Corporation ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit
Supplier: Genscript Corporation L00350C/ L00350CY

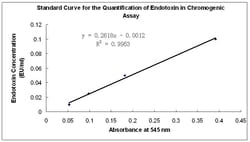
Genscript ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit is designed to be a quantitative In Vitro end-point endotoxin test for human and animal parenteral drugs, biological products, and medical devices.The system is not intended for detection of endotoxin in a licensed reagent, clinical samples or the diagnosis of human disease. A measurable endotoxin concentration range from 0.01 to 1 EU/ml can be achieved. Lyophilized Amebocyte Lysate reagent is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.