Learn More
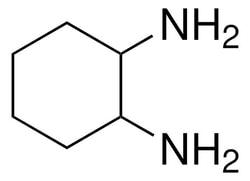
Sigma Aldrich 1,2-Diaminocyclohexane, mixture of cis and trans
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
- 1,2-diaminocyclohexane undergoes non-templated reaction with homochiral as well as the racemic form of trans-1,2-diaminocyclohexane with terephthaldehyde to yield (3+3)-cyclocondensed molecular triangles
- It acts as ligand and forms organotin complexes, having potential applications as metal-based antitumour drugs.1,2-diaminocyclohexane was used in the synthesis of chiral ruthenium(iv)-oxo complexes
Specifications
Specifications
| CAS | 694-83-7 |
| Density | 0.931 g/mL (at 25°C (literature)) |
| Boiling Point | 92°C to 93°C (18 mmHg) |
| Molecular Formula | C6H14N2 |
| Refractive Index | n20/D 1.49 (literature) |
| Linear Formula | C6H10(NH2)2 |
| MDL Number | MFCD00001491 |
| Quantity | 18 L |
| Synonym | 1,2-Cyclohexanediamine; DHC 99 |
| Molecular Weight (g/mol) | 114.19 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.