Learn More
Description
- Chloromethyl pivalate reacts with sodium salt of sulbactam to yield sulbactam pivoxil
- It undergoes acylation reaction with 9-(2-phosphonylmethoxyethyl)adenine (pmea) to yield bis(pivaloyloxymethyl) pmea.chloromethyl pivalate was used in the synthesis of pivaloyloxy methyl ester of ofloxacin as prodrug
- It was used as the reagent during the synthesis of an isoindoline-annulated, tricyclic sultam library via microwave-assisted, continuous-flow organic synthesis
Specifications
Specifications
| CAS | 18997-19-8 |
| Density | 1.045 g/mL (at 25°C (literature)) |
| Boiling Point | 146°C to 148°C (lit.) |
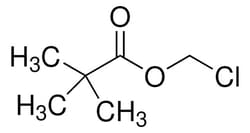
| Molecular Formula | C6H11ClO2 |
| Linear Formula | (CH3)3 CCOOCH2Cl |
| Refractive Index | n20/D 1.417 (literature) |
| MDL Number | MFCD00000884 |
| Quantity | 25 g |
| Synonym | POM-Cl; Pivaloyloxymethyl chloride |
| Molecular Weight (g/mol) | 150.6 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.