Learn More
R&D Systems™ Human Galectin-9 Quantikine ELISA Kit
Description
Specifications
Specifications
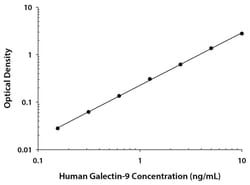
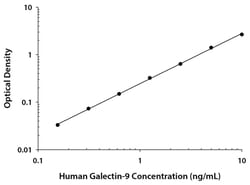
| Assay Range | 0.16 to 10 ng/mL |
| Assay Sensitivity | 0.028 ng/mL |
| Conjugate | HRP |
| Product Type | Solid Phase Sandwich ELISA |
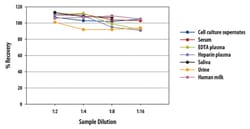
| Sample Type | Cell Culture Supernatant, Human Milk, Plasma (EDTA, Heparin), Saliva, Serum, Urine |
| Specificity | Natural and Recombinant Human Galectin-9 |
| For Use With (Application) | ELISA |
| Gene ID (Entrez) | 3965 |
| Gene Symbol | LGALS9 |
| Quantity | 1 Kit |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.