Learn More
Description
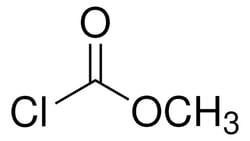
- Methyl chloroformate (mcf) is generally used for the derivatization of functional groups such as carboxylic acids, amines, and phenols.mcf can also be used:
- To activate 3-acylpyridines for nucleophilic addition with alkynyltin reagents to form 2,3-disubstituted 1,2-dihydropyridines
- Chloroesterification of terminal alkynes to form α-chloro-α,α-unsaturated esters
- As an electrophilic reagent to mediate the reaction of pyridine with lithium dialkyl- or diarylcuprates to form 4-substituted 1,4-dihydropyridine derivatives
- To convert nitronates into methoxycarbonyl nitronates
Specifications
Specifications
| CAS | 79-22-1 |
| Density | 1.223 g/mL (at 25°C (literature)) |
| Boiling Point | 70°C to 72°C (lit.) |
| Molecular Formula | C2H3ClO2 |
| Refractive Index | n20/D 1.387 (literature) |
| Linear Formula | ClCOOCH3 |
| MDL Number | MFCD00000639 |
| Quantity | 500 g |
| Synonym | MCF; Chloroformic acid methyl ester |
| Molecular Weight (g/mol) | 94.5 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.