Learn More
Description
- Methyl trifluoroacetate along with cesium fluoride or cesium chloride and cui constitutes a valuable trifluoromethylating reagent for substituting aromatic (or heteroaromatic) iodides and bromides
- Adsorption of methyl trifluoroacetate on amorphous silica has been investigated
- Fragmentation mechanism of generation of metastable ions from methyl trifluoroacetate has been investigated by mass analyzed ion kinetic energy spectra and ab initio molecular orbital calculations.methyl trifluoroacetate has been used as reagent for trifluoroacetylation of amines and amino acids
Specifications
Specifications
| CAS | 431-47-0 |
| Density | 1.273 g/mL (at 25°C (literature)) |
| Boiling Point | 43°C to 43.5°C (lit.) |
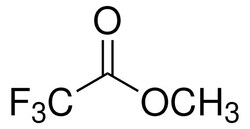
| Molecular Formula | C3H3F3O2 |
| Refractive Index | n20/D 1.291 (literature) |
| Linear Formula | CF3COOCH3 |
| MDL Number | MFCD00000417 |
| Quantity | 25 g |
| Molecular Weight (g/mol) | 128.05 |
| Percent Purity | 99% |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.