Learn More
Description
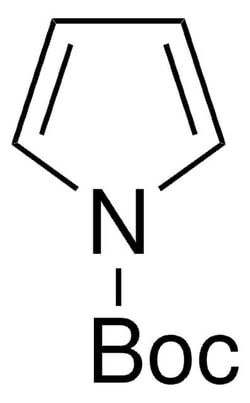
- N-boc-pyrrole is an n-protected pyrrole. it undergoes diels-alder reaction with enantiomerically pure allene-1,3-dicarboxylates to form endo-adducts with retention in configurations at two newly generated stereogenic centers. it also undergoes cyclopropanation with methyl phenyldiazoacetate to form both monocyclopropane and dicyclopropane. its IR-catalyzed c-h borylation followed by cross coupling with 3-chlorothiophene to form biheterocycle has been reported.n-boc-pyrrole was used in the synthesis of 1-(tert-butoxycarbonyl)-1h-pyrrol-2-ylboronic acid by treating with n-buli and subsequent reaction with trimethyl borate.it may be used as starting material in the synthesis of the following:
- Tropane drivatives
- N-boc-2-(4-methoxyphenyl)pyrrole
- N-boc-pyrrol-2-ylboronic acid
Specifications
Specifications
| CAS | 5176-27-2 |
| Density | 1 g/mL (at 25°C (literature)) |
| Boiling Point | 91°C to 92°C (20 mmHg) |
| Molecular Formula | C9H13NO2 |
| Refractive Index | n20/D 1.4685 (literature) |
| Linear Formula | C9H13NO2 |
| MDL Number | MFCD00209559 |
| Quantity | 100 mL |
| Synonym | tert-Butyl 1-pyrrolecarboxylate |
| Molecular Weight (g/mol) | 167.21 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.