Learn More
Description
- Triallylamine (taa) reacts with primary aromatic amines in the presence of a ruthenium catalyst to form 2-ethyl-3-methylquinolines
- Taa undergoes hydrozirconation followed by transmetalation with germanium tetrachloride to form 1-aza-5-germa-5-chlorobicyclo[3.3.3]undecane. this compound can react with grignard or lithium reagents to form the corresponding 5-organo compounds
- The cycloaddition of taa to fluorinated 1,3,4-oxadiazoles affords octahydro-2,7-methanofuro[3,2-c]pyridines
Specifications
Specifications
| CAS | 102-70-5 |
| Density | 0.79 g/mL (at 25°C (literature)) |
| Boiling Point | 150°C to 151°C (lit.) |
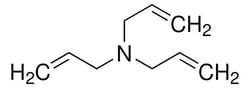
| Molecular Formula | C9H15N |
| Refractive Index | n20/D 1.451 (literature) |
| Linear Formula | (H2C=CHCH2)3 N |
| MDL Number | MFCD00026093 |
| Quantity | 250 mL |
| Synonym | TAA |
| Molecular Weight (g/mol) | 137.22 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.