Learn More
Description
- Conformational stability and vibrational IR and raman spectra of trichloroacetyl isocyanate has been investigated
- Trichloroacetyl isocyanate is commonly employed as in situ derivatizing reagent for the 13c NMR studies of alcohols, phenols and amines
- It undergoes 1,5-cycloaddition reaction with anhydro-2-deoxy-d-erythro- and -L-threo-pent-1-enitols and 1,5-anhydro-2-deoxy-d- and -L-arabino-hex-1-enitols to yield [2+2] and [4+2] cycloadducts.trichloroacetyl isocyanate was used in catalytic one-pot dehydrative glycosylation of 1-hydroxy carbohydrate
- It was also used as a reagent for the conversion of alcohols to carbamates
Specifications
Specifications
| CAS | 3019-71-4 |
| Density | 1.581 g/mL (at 25°C (literature)) |
| Boiling Point | 80°C to 85°C (20 mmHg) |
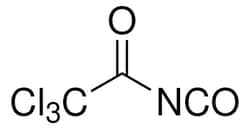
| Molecular Formula | C3Cl3NO2 |
| Refractive Index | n20/D 1.480 (literature) |
| Linear Formula | Cl3CCONCO |
| MDL Number | MFCD00002033 |
| Quantity | 1 g |
| Molecular Weight (g/mol) | 188.4 |
| Percent Purity | 96% |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.