Learn More
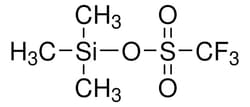
Sigma Aldrich Trimethylsilyl Trifluoromethanesulfonate
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
Trimethylsilyl trifluoromethanesulfonate has been used in combination with boron trifluoride etherate for the copper-catalyzed asymmetric allylic alkylation (AAA) of allyl bromides, chlorides, and ethers with organolithium reagents in the presence of a chiral ligand.It can be used:As a silylating agent for the synthesis of trimethylsilyl-enol ethers from esters of +a-diazoacetoacetic acid.To activate benzyl and allyl ethers for the alkylation of sulfides.To facilitate the conversion of Diels-Alder adducts of Danishefsky's diene to cyclohexenones without the formation of methoxy ketone by-product.To prepare difluoroboron triflate etherate, a powerful Lewis acid especially in acetonitrile solvent. As a reagent in a Dieckmann-like cyclization of ester-imides and diesters.It may also be used to catalyze:Acylation of alcohols with acid anhydrides.Reductive coupling of carbonyl compounds with trialkylsilanes to form symmetrical ethers.Glycosidation of 4-demethoxydaunomycinones with 1-O-acyl-L-daunosamine derivatives.
Specifications
Specifications
| CAS | 27607-77-8 |
| Density | 1.228 g/mL at 25°C |
| Boiling Point | 77°C80 mmHg, lit.) |
| Molecular Formula | C4H9F3O3SSi |
| Refractive Index | n20/D 1.36 |
| Linear Formula | CF3SO3Si(CH3)3 |
| MDL Number | MFCD00000406 |
| Quantity | 50 g |
| Synonym | TMS triflate; TMSOTf; Trifluoromethanesulfonic acid trimethylsilylester |
| Molecular Weight (g/mol) | 222.26 g/mol |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.