Learn More
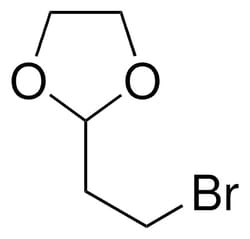
Sigma Aldrich 2-(2-Bromoethyl)-1,3-dioxolane
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
- Copper-catalyzed borylation of 2-(2-bromoethyl)-1,3-dioxolane with bis(pinacolato)diboron followed by treatment with potassium bifluoride affords the key organotrifluoroborate reagent.2-(2-bromoethyl)-1,3-dioxolane was used:
- As starting reagent for the synthesis of (5z,9z)-5,9-hexadecadienoic acid, (5z,9z)-5,9-nonadecadienoic acid and (5z,9z)-5,9-eicosadienoic acid
- In synthesis of 1-deoxy-castanospermine and 1-deoxy-8a-epi-castanospermine
- In asymmetric total synthesis of both enantiomers of marine mollusk metabolite puloαupone via evansα asymmetric diels-alder reaction
- As alkylating agent for amines, dithianes and carboximides
- With eynamides and sodium azide in a one-pot synthesis of triazoles
Specifications
Specifications
| CAS | 18742-02-4 |
| Density | 1.542 g/mL (at 25°C (literature)) |
| Boiling Point | 68°C to 70°C (8 mmHg) |
| Molecular Formula | C5H9BrO2 |
| Refractive Index | n20/D 1.479 (literature) |
| Linear Formula | C5H9BrO2 |
| MDL Number | MFCD00003216 |
| Quantity | 50 g |
| Synonym | 3-Bromopropionaldehyde ethylene acetal |
| Molecular Weight (g/mol) | 181.03 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.