Learn More
Cayman Chemical 4hydroxy Nonal MRcapturIc 10mg


Supplier: Cayman Chemical 3211010 MG

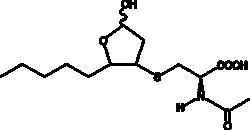
Peroxidation of common ω-6 PUFAs such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE. 4-HNE is cleared rapidly from the plasma and undergoes enterohepatic circulation as a glutathione conjugate in the rat.{7334} About two thirds of an administered dose of 4-HNE is excreted within 48 hours in the urine, primarily in the form of mercapturic acid conjugates.{8626} The C-1 aldehyde of 4-HNE is reduced to an alcohol in about half of these metabolites. The remainder are C-1 aldehydes or have been oxidized to C-1 carboxylic acids. These aldehydes and carboxylic acids can also form γ-lactols and γ-lactones, respectively, producing at least 4 or 5 end urinary metabolites of 4-HNE in vivo.
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.