Learn More
Description
Akt [also known as PKB (Protein kinase B) or RAC-PK (Related to the A and C kinases)] is a family of serine/threonine kinases that contains a pleckstrin homology (PH) domain. PH domains play important roles in signal transduction. There are three known iso forms of Akt in mammalian cells [Akt1 (α), Akt2 (β) and Akt3 (γ)]; they are thought to be regulated similarly. Akt is activated by insulin and growth factors by a mechanism involving phosphoinositide 3-OH kinase. Phosphoinositide 3-OH kinases products bi nd to the PH domain, resulting in translocation of Akt to the plasma membrane and activation of Akt to phospho-Akt by upstream kinases. Akt is phosphorylated within the activation loop at threonine 308 and the C-terminus at serine 473 (S473). Phospho-Akt promotes cell survival by inhibiting apoptosis. Specifically, phospho-Akt1 has been shown to phosphorylate Bad, a member of the Bcl-2 family that promotes cell death. This phosphorylation results in the inactivation of the proapoptotic function of Bad. The Akt molecule is thus considered to link extracellular survival signals (growth factors) with the apoptotic machinery (BAD). Akt is also a key mediator of the metabolic effects of insulin. Additionally, Akt has been referred to as an oncogene because it has increased activity in a number of tumors.
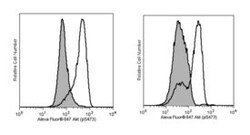
The M89-61 antibody recognizes Akt phosphorylated at S473. This phosphorylation site is shared by all three isoforms of Akt. The homologous phosphorylation sites in Akt2 and Akt3 are S474 and S472, respectively.

Specifications
Specifications
| Antigen | Akt (pS473) |
| Applications | Flow Cytometry |
| Classification | Monoclonal |
| Clone | M89-61 |
| Conjugate | Alexa Fluor 647 |
| Description | Akt1, Akt2, Akt3, PKBα, PKBβ, PKBγ, RAC-PKα, RAC-PKβ, RAC-PKγ, STK-2 |
| Formulation | Aqueous buffered solution containing BSA and ≤0.09% sodium azide. |
| Host Species | Mouse |
| Immunogen | Phosphorylated Human Akt1 (pS473) Peptide |
| Purification Method | Affinity Purified |
| Show More |
Safety and Handling
For Research Use Only.
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.