Learn More
KRISHGEN BIOSYSTEMS Immunoassay for the estimation of Adalimumab in human serum and plasma. Validated against ICH M10. Can be used for generics and biosimilars PK studies.
Supplier: KRISHGEN BIOSYSTEMS KBI1015

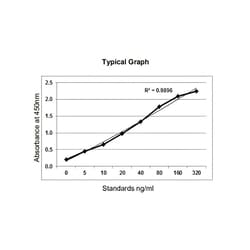
Pack Size: 1 x 96 wells. Background: Adalimumab is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor alpha (TNF alpha). Adalimumab is produced by recombinant DNA technology in a mammalian cell expression system and is purified by a process that includes specific viral inactivation and removal steps. Elevated levels of TNF are found in the synovial fluid of rheumatoid arthritis, including juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis patients and play an important role in both the pathologic inflammation and the joint destruction that are hallmarks of these diseases. Assay Range: 5 ng/ml - 320 ng/ml; Sensitivity: 4.8 ng/ml. This mAb-based Adalimumab PK ELISA detects adalimumab in human samples. Additional species validation available upon request. ELISA Features include a pre-coated plate with break-apart wells, standardisation and Lot to Lot Consistency with Precision CV <15%.
The Fisher Scientific Encompass Program offers items which are not part of our distribution portfolio. These products typically do not have pictures or detailed descriptions. However, we are committed to improving your shopping experience. Please use the form below to provide feedback related to the content on this product.