Learn More
Zinc chloride hydrate, Puratronic™, 99.999% (metals basis)
Description
It is used for a source of the zinc ion. It can also be used alone or with phenols for preserving railway ties, fireproofing timber, and with ammonium chloride as a flux for soldering and etching of metals. It is used as a moderate-strength lewis acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.

Specifications
Specifications
| Color | White |
| Physical Form | Crystalline Aggregates |
| Quantity | 250 g |
| Linear Formula | ZnCl2·xH2O |
| UN Number | UN3260 |
| Merck Index | 14,10132 |
| Solubility Information | Soluble in water,ethanol (slightly),acetone (slightly),ether,and glycerol. |
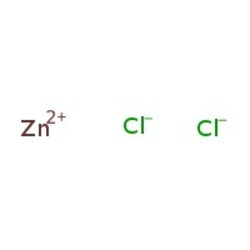
| SMILES | [Cl-].[Cl-].[Zn++] |
| IUPAC Name | zinc(2+) dichloride |
| Formula Weight | 136.29 (Anhydrous) |
| Show More |
Safety and Handling
| Hazard Category | H302-H314-H335 |
| Hazard Statement | GHS H Statement H314-H318-H302 Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if swallowed. |
| Precautionary Statement | P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c |
| DOTInformation | Transport Hazard Class: 8; Packing Group: III; Proper Shipping Name: CORROSIVE SOLID, ACIDIC, INORGANIC, N.O.S. |
| EINECSNumber | 231-592-0 |
| TSCA | Yes |
| Recommended Storage | Ambient temperatures |
RUO – Research Use Only
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.