Learn More
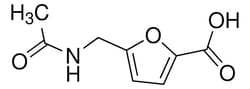
Sigma Aldrich 5-(acetamidomethyl)furan-2-carboxylic acid
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
- Diethyl chloromalonate (diethyl α-chloromalonate) is a 2-halo-1,3-dicarbonyl compound
- It participates in k2co3-catalyzed domino reactions (michael alkylation, mannich alkylation, and aldol alkylation) of salicylic aldehyde derivatives to afford functionalized 2,3-dihydrobenzofurans
- It reacts with cs2co3 in the presence of elemental s8 or sen to afford the corresponding diethyl thioxo- or selenoxomalonates, which can be trapped in situ with various 1,3-dienes
Specifications
Specifications
| Molecular Formula | C8H9NO4 |
| Linear Formula | C8H9NO4 |
| MDL Number | MFCD13187985 |
| Quantity | 25 mL |
| Molecular Weight (g/mol) | 183.16 |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.