Learn More
LCB1 Mouse, Unlabeled, Clone: 49, BD
Mouse Monoclonal Antibody
Supplier: BD Biosciences 611305
Description
Sphingolipid biosynthesis is initiated by condensation of L-serine with palmitoyl coenzyme A, a reaction catalyzed by serine palmitoyltransferase (SPT). SPT is the rate-determining enzyme in the sphingolipid pathway. This enzyme is a key component for regulating cellular sphingolipid content. Initially identified in SPT-deficient S. cerevisiae strains, LCB1 and LCB2 homologs have been identified and characterized in mouse, human, and CHO cell lines. The mammalian LCB1 protein has 35% amino acid identity with yeast LCB1, while the
mammalian LCB2 protein has 43% amino acid identity with yeast LCB2. Both LCB1 and LCB2 are transmembrane proteins containing protein localization sites, predicting they are membrane-bound enzymes enriched in the endoplasmic reticulum. In mouse tissue, LCB1 and LCB2 are expressed ubiquitously, with the highest levels detected in kidney and brain. Transfection of SPT-defective CHO mutant strains with LCB1-expressing plasmid restores both SPT activity and de novo sphingolipid synthesis to wild type levels. Thus, LCB1 may be an essential component of SPT activity during mammalian sphingolipid biosynthesis.
Host Species: Mouse
Clone: 49
Isotype: IgG1
Species Reactivity: Rat
Immunogen: Mouse LCB1 aa. 121-238
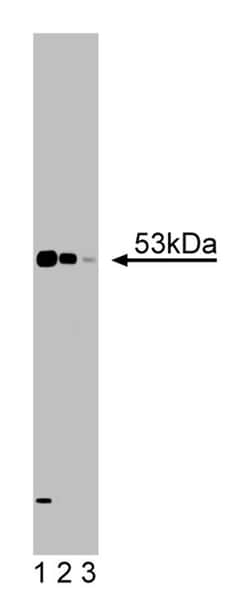
Formula Weight [Chemical]: 53kDa
Immunofluorescence, Western Blotting

Specifications
| LCB1 | |
| Monoclonal | |
| 250μg/mL | |
| Aqueous buffered solution containing BSA, glycerol, and ≤0.09% sodium azide. | |
| Mouse LCB1 aa. 121-238 | |
| 150 μg | |
| Cell Biology | |
| Human, Mouse, Rat | |
| IgG1 |
| Western Blot | |
| 49 | |
| Unconjugated | |
| Mouse | |
| Affinity Purified | |
| RUO | |
| Primary | |
| Store undiluted at -20°C. |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
For Research Use Only.