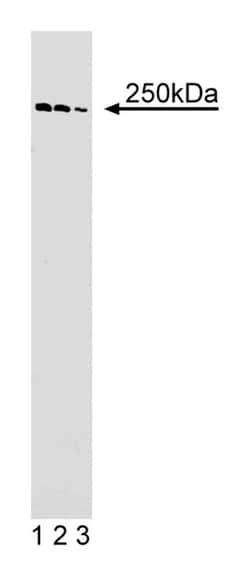Learn More
α-Spectrin II Mouse, Unlabeled, Clone: 35, BD
Mouse Monoclonal Antibody
Specifications
| Antigen | α-Spectrin II |
|---|---|
| Clone | 35 |
| Concentration | 250μg/mL |
| Applications | Immunofluorescence, Western Blot |
| Classification | Monoclonal |
Description
Spectrins are central components of the cytoskeleton that form a scaffold below the plasma membrane. Spectrins contain two subunits, α and β, which intertwine to form heterodimers that can self associate into elongated tetramers. α-spectin I and β-spectrin I form heterodimers in red blood cells, while nonerythroid mammalian cells contain heterodimers of α-spectin I and II with β-spectrin I to V. The structure of spectrins includes a succession of triple-helical repeats along with various domains, such as SH3 domain, EF hands, PH domains, and binding domains for ankyrin, actin, band 4.1, and calmodulin. α-spectrin II is a widely expressed non-erythroid α-spectrin that contains an SH3 domain, a calmodulin binding site, and two cleavage sites for proteases, such as calpains and caspase-3. β-spectrin II is a widely expressed non-erythroid β-spectrin that contains a C-terminal region that interacts with α-spectrins and a PH domain. α-spectrin II and β-spectrin II, like many other spectrins, can form heterodimers that can self associate into tetramers, as well as interact with Band 4.1, F-actin, and other proteins near the plasma membrane. This scaffold of cytoskeletal and plasma membrane proteins is critical for the maintenance of cell structure.
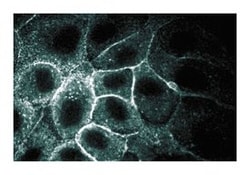
Immunofluorescence, Western Blotting
Specifications
| α-Spectrin II | |
| 250μg/mL | |
| Monoclonal | |
| Mouse | |
| Cell Biology | |
| Aqueous buffered solution containing BSA, glycerol, and ≤0.09% sodium azide. | |
| IgG1 | |
| Affinity Purified |
| 35 | |
| Immunofluorescence, Western Blot | |
| Unconjugated | |
| RUO | |
| Canine, Chicken, Human | |
| Human α-Spectrin II aa. 252-371 | |
| Primary |
For Research Use Only.
Your input is important to us. Please complete this form to provide feedback related to the content on this product.