Learn More
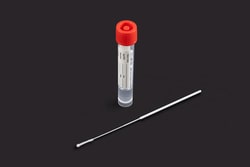
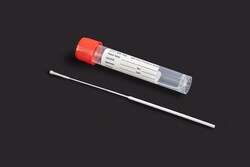
Great Lakes Diagnostics ARX Viral Transport Media
Description
INTENDED USE:
The ARX Liquid Amies Collection and Transport System is intended for use in the collection of clinical specimens (i.e., nasal secretion/wash; lachrymal secretion/tears; auricular secretion/cerumen; urethral, rectal, or vaginal swab; wound/abscess material) potentially containing aerobic, anaerobic, and fastidious bacteria and their transport at 2 to 8°C or 25 to 30°C from the patient to the laboratory for bacteriological examination and culture. In the laboratory, the collected and transported clinical specimens of nasal, lachrymal, ceruminous, vaginal, urethral, rectal, and wound/abscess origin are processed using standard clinical laboratory operating procedures for bacterial culture.
SUMMARY AND EXPLANATION
Specialized systems for collecting and transporting bacteriological specimens are commonly used in laboratories to aid in the diagnosis of bacterial infections, especially when there is a delay between specimen collection and processing. The ARX Liquid Amies Collection and Transport System consists of a sterile a pre-scored Microbrush™ Test Swab, and a polypropylene screw-cap vial containing 1 mL of modified liquid Amies medium. Microbrush™ Test Swab flocked swabs are available in various score points and configurations to facilitate specimen collection from various sites on patients' bodies.
PRINCIPLES OF THE PROCEDURE
Once a specimen is collected with a swab, and added to the transport medium vial, it is immediately processed to achieve optimal recovery. For cases where immediate processing (i.e., within 2 hours) is not possible, specimens can be stored at 2 to 30°C and processed within 48 hours (except for Neisseria gonorrhea, which should be processed within 24 hours). Recent independent studies suggest that the viability of certain bacteria in swab transport systems will improve when transported or stored at refrigerated temperature.
Specifications
Specifications
| Product Type | Viral Transport Media |
| Format | Tube |
| Sterility | Sterile |
| Swab Configuration | Not Applicable |
| Certifications/Compliance | FDA |
| Closure Color | Red |
| Closure Type | Screw Cap |
| Color | Clear |
| Diameter (Metric) | 90 mm |
| Fill Volume | 1 mL |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.