Learn More
Sigma Aldrich C5 Lenalidomide-Dipiperazine-Nh2 Hydrochloride
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
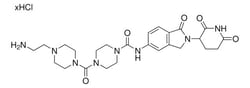
Protein degrader building block Thalidomide-Photoswitch3-NH2 hydrochloride enables the synthesis of PHOtochemically TArgeting Chimeras (PHOTACs), or photoswitchable proteolysis-targeting chimeras (PROTACs) that can be reversibly activated with different wavelengths of light. Developed in the Trauner and Pagano labs, PHOTACs are inactive in the dark but are activated to the cis isomer via irradiation at 390 nm and reversibly deactivated at wavelengths above 450 nm. As described in Reynders et al, this conjugate was used to prepare PHOTAC-I-10 and PHOTAC-II-6 and contains a Cereblon (CRBN)-recruiting ligand, an azobenzene photoswitchable crosslinker, and a pendant amine for reactivity with an acid on the target warhead. Light-mediated control of the resulting PHOTAC affords advanced temporal and spatial control of targeted protein degradation. Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm. Low-intensity light needed for photoactivation is not cytotoxic. Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
Learn more: Technology Spotlight: Degrader Building Blocks for Targeted Protein DegradationPortal: Building PROTAC Degraders for Targeted Protein DegradationPHOTACs enable optical control of protein degradationPhotoPROTACs: A Novel Biotechnology for Cancer TreatmentPROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
Specifications
Specifications
| CAS | 9375-5-4-0 |
| Quantity | 50 mg |
| Synonym | 2-(2-((6-Aminohexyl)oxy)ethoxy)-N-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)acetamide hydrochloride; C5 Lenalidomide conjugate; Crosslinker-E3 Ligase ligand conjugate; Protein degrader building block for PROTAC(R) research; Template for synthesis of targeted protein degrader |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.