Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Invitrogen™ Caplacizumab Humanized Recombinant Human Monoclonal Antibody
Human Recombinant Monoclonal Antibody
Supplier: Invitrogen™ MA541954
This item is not returnable.
View return policy
Description
Endotoxin level < 0.01EU/μg by LAL method Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 °C for one week. Store at -20 to -80 °C for twelve months from the date of receipt.
Caplacizumab, firstly called ALX-0081, is a humanized single-variable-domain immunoglobulin consisting of two identical humanized building blocks genetically linked by a three-alanine linker. Caplacizumab was developed by Ablynx, a Sanofi company and FDA approved on February 6, 2019, 6and approved previously by the EU in October 2018 as a combination therapy with plasma exchange and immunosuppression.
Specifications
| Caplacizumab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| ALX-0081; caplacizumab-yhdp; PMP12A2h1-linker AAA-PMP12A2h1; VWF A1 domain | |
| Human VWF A1 domain. | |
| 100 μg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Liquid |
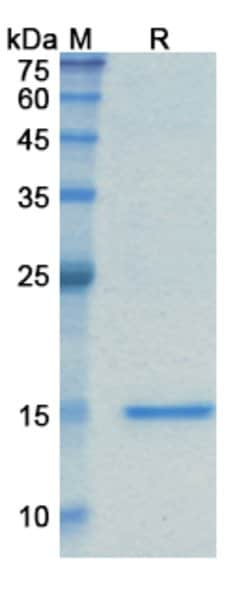
| ELISA, SDS-Page | |
| 1.6 mg/mL | |
| 20mM citric acid with 6.2% sucrose, 0.01% Tween 80 and no preservative | |
| Human | |
| Protein A/G | |
| RUO | |
| Human | |
| Antibody | |
| Chemical |
Safety and Handling
WARNING: Cancer - www.P65Warnings.ca.gov
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction