Learn More
Daratumumab Mouse, Clone: 11F2, GenScript™
Mouse Monoclonal Antibody
Supplier: Genscript Corporation A0199640

Description
Reconstitute the lyophilized antibody with deionized water (or equivalent) to a final concentration of 0.5 mg/mL. Daratumumab (Darzalex) is a human monoclonal antibody that is approved by the U.S. Food and Drug Administration for the treatment of multiple myeloma. Daratumumab binds to CD38 on multiple myeloma cells and induces cells to apoptose via antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity.
GenScript Anti-Daratumumab Antibody (11F2), mAb, Mouse is produced from a hybridoma resulting from the fusion of partner and B-lymphocytes obtained from a mouse immunized with Daratumumab.
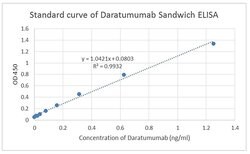
Daratumumab Monoclonal antibody specifically detects Daratumumab in Chemical samples. It is validated for ELISASpecifications
| Daratumumab | |
| Monoclonal | |
| Unconjugated | |
| Darzalex | |
| Daratumumab | |
| 40 μg | |
| Primary | |
| Antibody | |
| IgG1 |
| ELISA | |
| 11F2 | |
| PBS with 0.02% sodium azide; pH 7.4 | |
| Mouse | |
| Protein A | |
| RUO | |
| -20°C or -80°C if preferred | |
| Lyophilized | |
| Chemical |
The Fisher Scientific Encompass Program offers items which are not part of our distribution portfolio. These products typically do not have pictures or detailed descriptions. However, we are committed to improving your shopping experience. Please use the form below to provide feedback related to the content on this product.