Learn More
Invitrogen™ HSD3B1 Monoclonal Antibody (3F3C/7F9C8)


Mouse Monoclonal Antibody
Supplier: Invitrogen™ MA549243
Description
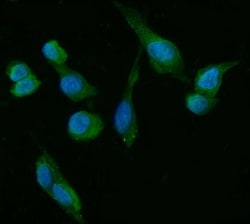
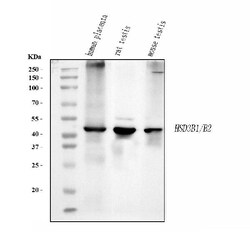
Adding 0.2 mL of distilled water will yield a concentration of 500 μg/mL. Positive Control - WB: human placenta tissue, rat testis tissue, mouse testis tissue. ICC/IF: U20S cell. Store at -20°C for one year from date of receipt. After reconstitution, at 4°C for one month. It can also be aliquotted and stored frozen at -20°C for six months. Avoid repeated freeze-thaw cycles.
Amyloid beta peptide (Abeta/Beta-amyloid) is the major constituent of amyloid plaques in the brains of individuals afflicted with Alzheimer's disease. Abeta peptide is 40-43 amino acids long and generated from the beta-amyloid precursor protein (beta APP) in a two-step process. The first step involves cleavage of the extracellular, amino-terminal domain of beta APP. Protein cleavage is performed by an aspartyl protease, beta-secretase (BACE) which is synthesized as a propeptide and must be modified to the mature and active form by the prohormone convertase, furin. Beta APP cleavage by the mature form of BACE results in the cellular secretion of a segment of beta APP, and a membrane-bound remnant. The remnant protein is processed by another protease, gamma-secretase. Gamma-secretase cleaves an intra-membrane site in the carboxyl-terminal domain of beta APP, thus generating the amyloid beta peptide. Gamma-secretase is believed to be a multi-subunit complex containing presenilin-1 and 2 as central components. The transmembrane glycoprotein, nicastrin, is associated with presinilins and has been found to bind to the carboxyl-terminus of beta APP and helps to modulate the production of the amyloid beta peptide. Abeta is an extracellular filamentous protein component of amyloid cores, neuritic plaques and is also found as a deposit in neurofibrillary tangles. Alzheimer's disease, the most common cause of senile dementia, is characterized by abnormal filamentous protein deposits in the brain. Beta amyloid deposits are also detected in Lewy body dementia, Down's syndrome, amyloidosis (Dutch type), cerebroarterial amyloidosis (cerebral amyloid angiopathy) and in the Guam Parkinson-Dementia complex.
Specifications
| HSD3B1 | |
| Monoclonal | |
| 500 μg/mL | |
| PBS with 4mg trehalose and no preservative | |
| P14060, P22071, P24815 | |
| HSD3B1 | |
| E.coli-derived human HSD3B1 recombinant protein (Position: Q17-Q373). | |
| 100 μg | |
| Primary | |
| Human, Mouse, Rat | |
| Antibody | |
| IgG2b |
| Western Blot, Immunocytochemistry | |
| 3F3C/7F9C8 | |
| Unconjugated | |
| HSD3B1 | |
| 3 beta-hydroxysteroid dehydrogenase 1; 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase; 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1; 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type I; 3BETAHSD; 3-beta-HSD; 3-beta-HSD I; 3-beta-hydroxy-5-ene steroid dehydrogenase; 3-beta-hydroxy-Delta(5)-steroid dehydrogenase; 3-beta-hydroxysteroid 3-dehydrogenase; 3BH; Delta-5-3-ketosteroid isomerase; Dihydrotestosterone oxidoreductase; HGNC:5217; HSD3B; Hsd3b1; HSDB3; HSDB3A; hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1; hydroxy-delta-MAY-steroid dehydrogenase, 3 beta- and steroid delta-isomerase; hydroxysteroid dehydrogenase-1, delta>5>-3-beta; I; Progesterone reductase; SDR11E1; short chain dehydrogenase/reductase family 11E, member 1; steroid Delta-isomerase; Trophoblast antigen FDO161G; unnamed protein product | |
| Mouse | |
| Antigen affinity chromatography | |
| RUO | |
| 15492, 3283, 360348 | |
| -20°C | |
| Lyophilized |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.