Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Human RANK/TNFRSF11A (Research Grade Denosumab Biosimilar) Antibody, Novus Biologicals™


Human Monoclonal Antibody
Supplier: R&D Systems MAB11492SP
This item is not returnable.
View return policy
Description
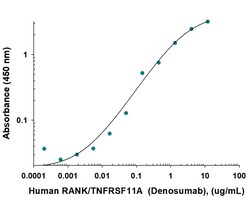
RANK/TNFRSF11A Monoclonal antibody specifically detects RANK/TNFRSF11A in Human samples. It is validated for ELISASpecifications
| RANK/TNFRSF11A | |
| Monoclonal | |
| Unconjugated | |
| Q9Y6Q6 | |
| Human | |
| Protein A or G purified from hybridoma culture supernatant | |
| RUO | |
| 8792 | |
| Human | |
| Purified |
| ELISA | |
| Hu200 | |
| Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details. *Small pack size (-SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. | |
| CD265, CD265 antigen, FEO, loss of heterozygosity, 18, chromosomal region 1, ODFR, ODFROSTS, OFE, OPTB7, Osteoclast differentiation factor receptor, PDB2, RANKLOH18CR1, Receptor activator of NF-KB, receptor activator of nuclear factor-kappa B, TNFRSF11A, TRANCE R, TRANCER, tumor necrosis factor receptor superfamily member 11A, tumor necrosis factor receptor superfamily, member 11a, activator of NFKB, tumor necrosis factor receptor superfamily, member 11a, NFKB activator | |
| Mouse myeloma cell line NS0-derived recombinant human RANK/TNFRSF11A, extracellular domain, Accession # Q9Y6Q6 | |
| 25 μg | |
| Primary | |
| Reconstitute at 0.5 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration. | |
| Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20 to -70 °C as supplied. 1 month, 2 to 8 °C under sterile conditions after reconstitution. 6 months, -20 to -70 °C under sterile conditions after reconstitution. | |
| IgG1 |
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction