Learn More
Description
Includes
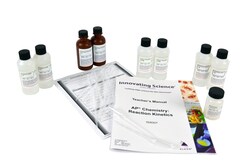
Materials for 15 groups: 100mL Potassium Iodide, 0.1% solution 0.006M (2); 100mL Sodium Thiosulfate, 0.001M solution (2); 100mL Hydrogen Peroxide, 3% solution 0.88M (2); 5 capsules Starch Soluble (5 caps per bottle); 100mL Hydrochloric Acid, 0.1M solution UN1789 (2); 150 pipets; teacher's guide; and student copy masters
By varying concentration of reactants, the rate law is determined. By varying the temperature, the activation energy is determined. The data should be graphed and analyzed during the lab so additional measurements can be made if necessary.
In a chemical reaction, reactants are converted to product at a given rate. This rate can be changed by alternating either the temperature of the reaction, the form of the reactants, the concentration of reactants or products, or by adding a catalyst.
Specifications
Specifications
| Color | White |
| Grade | 7 to 14 |
| Age | 13 to 20 |
| For Use With (Application) | By varying concentration of reactants, the rate law is determined, by varying the temperature, the activation energy is determined |
| Includes | 2 x 100mL Potassium Iodide, 0.1% solution (0.006M), 2 x 100mL Sodium Thiosulfate, 0.001M solution, 2 x 100mL Hydrogen Peroxide, 3% solution (0.88M), 5 capsules Starch soluble, 2 x 100mL Hydrochloric Acid, 0.1M solution UN1789 pipettes, teacher's guide and student study guide copymasters |
| Quantity | 1 |
| Dimensions (L x W x H) | 8 x 8 x 8 in. |
| Class Size | 15 Lab Groups |
| Product Type | Reaction Kinetics Chemistry Kit |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.