Learn More
Innovating Science™ AP Chemistry Kits: Water Hardness


Shop All Aldon Products


Description
Includes
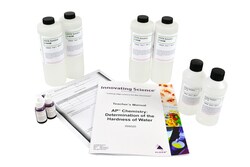
Materials for 15 groups: 500mL EDTA solution, 0.005M (4); 200mL Buffer solution (2); 15mL EBT (Eriochrome Black, 0.1%) solution UN 1219 (2); teacher's guide; and student copy masters
Tap water contains various amounts of ions such as calcium, iron, magnesium, etc. The higher the concentration, the “harder” the water is; hard water is characterized by the inability of soap to form lather and the formation of “scum” in sinks and bathtubs.
A sample of tap water is treated with EBT indicator. If the indicator turns from blue to pink, metal ions such as calcium and magnesium are present. To determine the concentration of ions present, the sample is titrated with a known molar concentration of EDTA.
Specifications
Specifications
| Certifications/Compliance | 49 CFR 173.4 |
| Color | White |
| Grade | 7 to 14 |
| Age | 13 to 20 |
| For Use With (Application) | Used to signal the presence of ions in the water sample |
| Includes | 4 x 500ml 0.005m edta solution, 2 x 200ml buffer solution, 2 x 15ml ebt solution (eriochrome black, 0.1%), teacher's guide and student study guide copymasters |
| Quantity | 1 |
| Dimensions (L x W x H) | 7.25 x 7.25 x 8.375 in. |
| Class Size | 15 Lab Groups |
| Product Type | Water Hardness Chemistry Kit |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.