Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Invitrogen™ Inotuzumab Humanized Recombinant Human Monoclonal Antibody


Human Recombinant Monoclonal Antibody
Supplier: Invitrogen™ MA541854
This item is not returnable.
View return policy
Description
Endotoxin level < 0.01EU/μg by LAL method Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 °C for one week. Store at -20 to -80 °C for twelve months from the date of receipt.
Inotuzumab ozogamicin is an antibody-pharmaceutical conjugate using linker and cytotoxic pharmaceutical technology similar to that developed for the ground-breaking treatment Mylotarg (Gemtuzumab ozogamicin), which was approved by the US FDA in 2000 for the treatment of acute myeloid leukemia. Inotuzumab ozogamicin consists of a recombinant humanized IgG4 kappa CD22-targeting monoclonal antibody covalently attached to calicheamicin derivative, N-acetyl-gamma-calicheamicin dimethylhydrazide, which is a potent DNA-binding cytotoxic agent 4. Developed by Pfizer and UCB, inotuzumab ozogamicin was granted approval by EU in June 2017 followed by FDA on August 17th, 2017 for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). ALL is a rapidly progressing cancer of the bone marrow that is associated with high mortality rates and low therapeutic response from standard chemotherapies in relapsing conditions. In a randomized trial, inotuzumab ozogamicin displayed higher percentages of patients undergoing longer periods of complete remission with no evidence of disease in comparison to patients receiving alternative chemotherapy 5.
Specifications
| Inotuzumab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| CMC-544 | |
| Human CD22. | |
| 100 μg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Liquid | |
| Chemical |
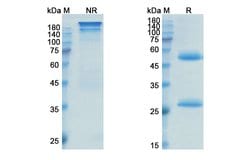
| ELISA, SDS-Page | |
| 2.7 mg/mL | |
| PBS with no preservative; pH 7.4 | |
| Human | |
| Protein A/G | |
| RUO | |
| Human | |
| Antibody | |
| IgG4 κ |
Safety and Handling
WARNING: Cancer - www.P65Warnings.ca.gov
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction