Learn More
Description
- Iodopentafluorobenzene forms supramolecular complexes with aromatic electron donors by forming halogen bonds to form discrete heterodimeric aggregates
- Iodopentafluorobenzene was used to study the formation of radical ions of iodopentafluorobenzene in aqueous solution
- It was used as solvent in a study to determine singlet oxgen lifetimes from phosphorescence decays in halogen substituted perfluorinated solvents by infrared emission spectrometery
- It has potential applications in plasma processing industry and in preparation of catalysts
Specifications
Specifications
| CAS | 827-15-6 |
| Density | 2.204 g/mL (at 25°C (literature)) |
| Boiling Point | 161°C (lit.) |
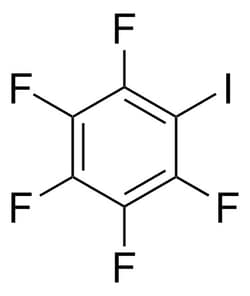
| Molecular Formula | C6F5I |
| Refractive Index | n20/D 1.496 (literature) |
| Linear Formula | C6F5I |
| MDL Number | MFCD00001032 |
| Quantity | 5 g |
| Molecular Weight (g/mol) | 293.96 |
| Percent Purity | 99% |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.