Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Invitrogen™ Palivizumab Humanized Recombinant Human Monoclonal Antibody
Human Recombinant Monoclonal Antibody
Supplier: Invitrogen™ MA541718
This item is not returnable.
View return policy
Description
Endotoxin level < 0.01EU/μg by LAL method Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 °C for one week. Store at -20 to -80 °C for twelve months from the date of receipt.
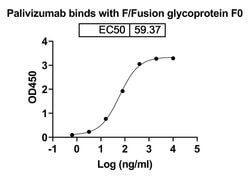
Palivizumab with trade name Synagis, is an FDA-approved drug for the prevention of respiratory syncytial virus (RSV) infections. It is a recombinant humanized IgG1 kappa isotype monoclonal antibody. Palivizumab binds the RSV envelope fusion protein (RSV F) on the surface of the virus and blocking a critical step in the membrane fusion process.
Specifications
| Palivizumab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| MEDI-493; Synagis | |
| Respiratory syncytial virus- RSV. | |
| 100 μg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Liquid | |
| Chemical |
| ELISA, SDS-Page | |
| 2.47 mg/mL | |
| PBS with no preservative; pH 7.4 | |
| Human | |
| Protein A/G | |
| RUO | |
| Virus | |
| Antibody | |
| IgG1 κ |
Safety and Handling
WARNING: Cancer - www.P65Warnings.ca.gov
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction