Learn More
Prednisolone Acetate Pharmaceutical Secondary Standard, MilliporeSigma™ Supelco™
Shop All MilliporeSigma Supelco ProductsDescription

Specifications
Specifications
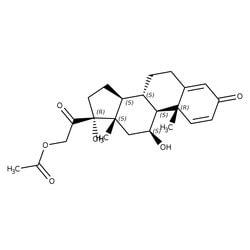
| CAS | 52-21-1 |
| Purity Grade Notes | These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available. |
| Linear Formula | C23H30O6 |
| MDL Number | MFCD00037710 |
| Synonym | Prednisolone 21-acetate; 1,4-Pregnadiene-11 beta,17 alpha,21-triol-3,20-dione 21-acetate; 11 beta,17 alpha,21-Trihydroxy-1,4-pregnadiene-3,20-dione 21-acetate; 21-Acetoxy-1,4-pregnadiene-11 beta,17 alpha-diol-3,20-dione |
| Type | Certified Reference Material |
| Quantity | 1 g |
| Formula Weight | 402.48 |
| Grade | Certified Reference Material |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.