Learn More
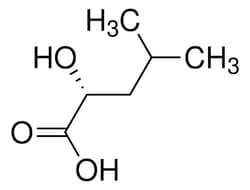
Sigma Aldrich (R)-2-hydroxy-4-methylpentanoic acid
Shop All MilliporeSigma Sigma Organic Chemistry Products
Description
- 1-acetoxy-1,3-butadiene (1-abd) can be generated by potassium or sodium acetate catalyzed reaction between crotonaldehyde and acetic anhydride. the product is a mixture of cis and trans forms, that has been confirmed by its physical properties and IR spectra. it is reported to be formed as a major product during the acetoxylation of 1,3-butadiene (bd) in gas-phase in the presence of pd-koac (palladium-potassium acetate) catalyst. 1-abd participates as 2+ß or 4+ß diene in cycloaddition reactions. enantioselective diels alder reaction of 2-methoxy-5-methyl-1,4-benzoquinone with 1-abd in the presence of molecular sieves (mesh size:4+a) has been reported.1-acetoxy-1,3-butadiene was used as diene in the following reactions:
- Diels-alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone
- Diels-alder reaction with diethyl ketovinylphosphonate, with and without lewis acid assistance
- Diels-alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid. it was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2h-azirine-3-carboxylate and during visible light photocatalysis. it was also used as reactant during intermolecular oxa-pictet-spengler cyclization
Specifications
Specifications
| CAS | 20312-37-2 |
| Molecular Formula | C6H12O3 |
| Linear Formula | C6H12O3 |
| MDL Number | MFCD00211263 |
| Quantity | 10 g |
| Molecular Weight (g/mol) | 132.16 |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.