Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
R&D Systems™ Recombinant Human NKp65/KLRF2 Fc Chimera Protein
Click to view available options
Quantity:
50 μg
Description
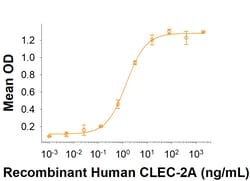
When Recombinant Human NKp65/KLRF2 Fc Chimera (Catalog # 10371-NK) is immobilized 0.1 μg the concentration of Recombinant Human CLEC-2A (Catalog # 8435-CL ) the optimal binding response is approximately 0.5-3 ng/mL.
Specifications
Specifications
| Accession Number | D3W0D1.1 |
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Gene ID (Entrez) | 100431172 |
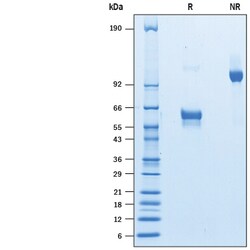
| Molecular Weight (g/mol) | Observed: 50-65 kDa, under reducing conditions |
| Quantity | 50 μg |
| Source | Chinese Hamster Ovary cell line, CHO-derived human NKp65/KLRF2 protein MD Human IgG1 (Pro100-Lys330) IEGR Human NKp65/KLRF2 (Asp52-Val156) Accession # D3W0D1.1(N-terminus) (C-terminus) |
| Storage Requirements | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20°C to -70°C as supplied. 1 month, 2°C to 8°C under sterile conditions after reconstitution. 3 months, -20°C to -70°C under sterile conditions after reconstitution. |
| Endotoxin Concentration | <0.10 EU / 1 μg of the protein by the LAL method. |
| Conjugate | Unconjugated |
| Reconstitution | Reconstitute at 200 μg/mL in PBS. |
| Show More |
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction