Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Invitrogen™ Risankizumab Humanized Recombinant Human Monoclonal Antibody


Human Recombinant Monoclonal Antibody
Supplier: Invitrogen™ MA542076
This item is not returnable.
View return policy
Description
Endotoxin level < 0.01EU/μg by LAL method Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 °C for one week. Store at -20 to -80 °C for twelve months from the date of receipt.
Risankizumab is a fully humanized IgG1 monoclonal antibody (mAb) that is selective for interleukin 23 (IL-23). On April 23, 2019 it was approved by the FDA (as SKYRIZI) for the treatment of moderate-to-severe plaque psoriasis in adults who are clinically considered eligible for systemic therapy or phototherapy for psoriasis. Label This pharmaceutical has been commercialized through collaboration between the German pharmaceutical company, Boehringer Ingelheim, and Abbvie.
Specifications
| Risankizumab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| BI 655066; risankizumab-rzaa | |
| Human IL23A. | |
| 100 μg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Liquid | |
| Chemical |
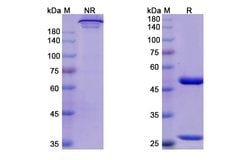
| ELISA, SDS-Page | |
| 1 mg/mL | |
| PBS with no preservative; pH 7.4 | |
| Human | |
| Protein A/G | |
| RUO | |
| Human | |
| Antibody | |
| IgG1 κ |
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction