Learn More
Description
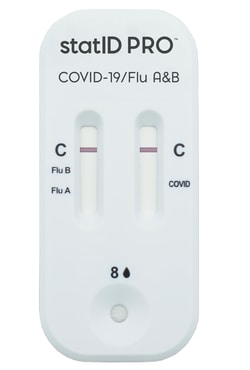
- One swab delivers antigen results for COVID-19, influenza A, and influenza B.
- FDA-cleared rapid test (not EUA), providing trusted regulatory approval.
- Approved for use in patients 2 years of age and older.
- Provides clear visual results in 15 minutes to support immediate clinical decision-making.
- Offers workflow flexibility, allowing specimens to be held in the extraction tube for up to 1 hour before testing.
Specifications
Specifications
| CE Marker | No |
| Clia Complexity | Waived |
| Detectable Analytes | COVID-19 |
| DoA Calibrators | No |
| Disposable | Single-use |
| Form | Ready-to-Use (RTU) |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.