Learn More
Invitrogen™ Golimumab Recombinant Human Monoclonal Antibody


Description
Endotoxin level < 0.01EU/μg by LAL method Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 °C for one week. Store at -20 to -80 °C for twelve months from the date of receipt.

Specifications
Specifications
| Antigen | Golimumab |
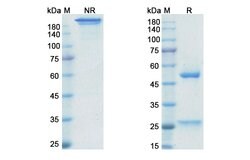
| Applications | ELISA, SDS-Page |
| Classification | Recombinant Monoclonal |
| Concentration | 2.8 mg/mL |
| Conjugate | Unconjugated |
| Formulation | PBS with no preservative; pH 7.4 |
| Gene Alias | CNTO 148; Simponi |
| Host Species | Human |
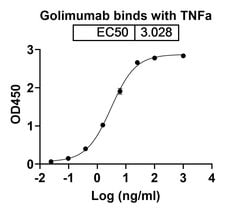
| Immunogen | Human TNFA/TNF alpha. |
| Purification Method | Protein A/G |
| Show More |
Safety and Handling
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.