Promotional price valid on web orders only. Your contract pricing may differ. Interested in signing up for a dedicated account number?
Learn More
Learn More
Human CD30/TNFRSF8 F(ab')2 (Research Grade Brentuximab Biosimilar) Antibody, Novus Biologicals™


Human Monoclonal Antibody
Supplier: R&D Systems MAB11129FAB2100
This item is not returnable.
View return policy
Description
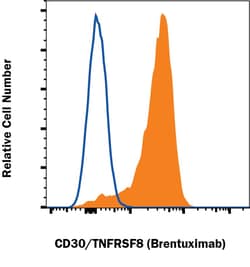
CD30/TNFRSF8 Monoclonal antibody specifically detects CD30/TNFRSF8 in Human samples. It is validated for Flow CytometrySpecifications
| CD30/TNFRSF8 | |
| Monoclonal | |
| Unconjugated | |
| Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. | |
| Human | |
| Protein A or G purified from cell culture supernatant | |
| RUO | |
| 943 | |
| Human | |
| Purified |
| Flow Cytometry | |
| Hu8F2 | |
| Flow Cytometry 0.25 ug/mL | |
| CD30, CD30 antigen, CD30KI-1, CD30L receptor, cytokine receptor CD30, D1S166EKi-1, Ki-1 antigen, Lymphocyte activation antigen CD30, TNFRSF8, tumor necrosis factor receptor superfamily member 8, tumor necrosis factor receptor superfamily, member 8 | |
| Human CD30/TNFRSF8 | |
| 100 μg | |
| Primary | |
| Reconstitute at 0.5 mg/mL in sterile PBS. | |
| Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20 to -70 °C as supplied. 1 month, 2 to 8 °C under sterile conditions after reconstitution. 6 months, -20 to -70 °C under sterile conditions after reconstitution. | |
| IgG1 |
Product Content Correction
Your input is important to us. Please complete this form to provide feedback related to the content on this product.
Product Title
Spot an opportunity for improvement?Share a Content Correction