Learn More
Ipilimumab Mouse, Clone: 26B6H7D9, GenScript™

Description
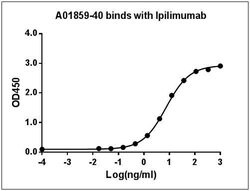
Reconstitute the lyophilized antibody with deionized water (or equivalent) to a final concentration of 0.5 mg/mL. Ipilimumab (Yervoy) is a humanized monoclonal antibody that is approved by the U.S. Food and Drug Administration for the treatment of patients with unresectable or metastatic melanoma. Ipilimumab works to activate the immune system by targeting CTLA-4 (Cytotoxic T-lymphocyte protein 4), a negative regulator of cytotoxic T-lymphocyte activity. Ipilimumab binds to CTLA-4, blocking the inhibitory signal, which allows the cytotoxic T-lymphocytes to destroy the cancer cells Anti-Ipilimumab Antibody (26B6H7D9), mAb, Mouse is produced from a hybridoma resulting from the fusion of partner and B-lymphocytes obtained from a mouse immunized with Ipilimumab.
Specifications
Specifications
| Antigen | Ipilimumab |
| Applications | ELISA |
| Classification | Monoclonal |
| Clone | 26B6H7D9 |
| Conjugate | Unconjugated |
| Formulation | PBS with 0.02% sodium azide; pH 7.4 |
| Gene Alias | Yervoy |
| Host Species | Mouse |
| Immunogen | Ipilimumab |
| Purification Method | Protein A |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.