Learn More
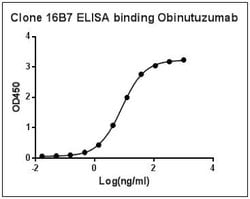
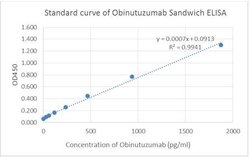
Obinutuzumab Mouse, Clone: 16B7, GenScript™

Description
Reconstitute the lyophilized antibody with deionized water (or equivalent) to a final concentration of 0.5 mg/mL. Obinutuzumab is a humanized anti-CD20 monoclonal antibody, originated by GlycArt Biotechnology AG and developed by Roche as a cancer treatment. It was approved under the trade name Gazyva by the US FDA in 2013, and as Gazyvaro by the EMA in Europe, for the treatment of chronic lymphocytic leukemia in combination with chemotherapy in treatment-naive patients, and as a second line treatment for follicular lymphoma. Obinutuzumab is a fully humanized monoclonal antibody that binds to an epitope on CD20 that partially overlaps with the epitope recognized by rituximab. Obinutuzumab binds to CD20 on B cells and causes these cells to be destroyed by engaging the adaptive immune system, directly activating intracellular apoptosis pathways, and activating the complement system.
Specifications
Specifications
| Antigen | Obinutuzumab |
| Applications | ELISA |
| Classification | Monoclonal |
| Clone | 16B7 |
| Conjugate | Unconjugated |
| Formulation | PBS with 0.02% sodium azide; pH 7.4 |
| Gene Alias | Gazyva; Gazyvaro |
| Host Species | Mouse |
| Immunogen | Obinutuzumab |
| Purification Method | Protein A |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.