Learn More
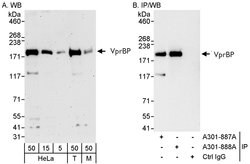
VprBP Rabbit anti-Human, Mouse, Polyclonal, Bethyl Laboratories

Description
The recommended shelf life for this product is 1 year from date of receipt.
Specifications
Specifications
| Antigen | VprBP |
| Applications | Immunoprecipitation, Western Blot |
| Classification | Polyclonal |
| Concentration | 0.2 mg/ml |
| Conjugate | Unconjugated |
| Formulation | TBS with 0.1% BSA and 0.09% sodium azide |
| Gene | DCAF1 |
| Gene Accession No. | Q80TR8, Q9Y4B6 |
| Gene Alias | DCAF1, DDB1 and CUL4 associated factor 1, DDB1- and CUL4-associated factor 1, HIV-1 Vpr-binding protein, KIAA0800, MGC102804, serine/threonine-protein kinase VPRBP, Vpr (HIV-1) binding protein, Vpr-binding protein, VPRBP, vpr-interacting protein |
| Gene Symbols | DCAF1 |
| Show More |
By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy.